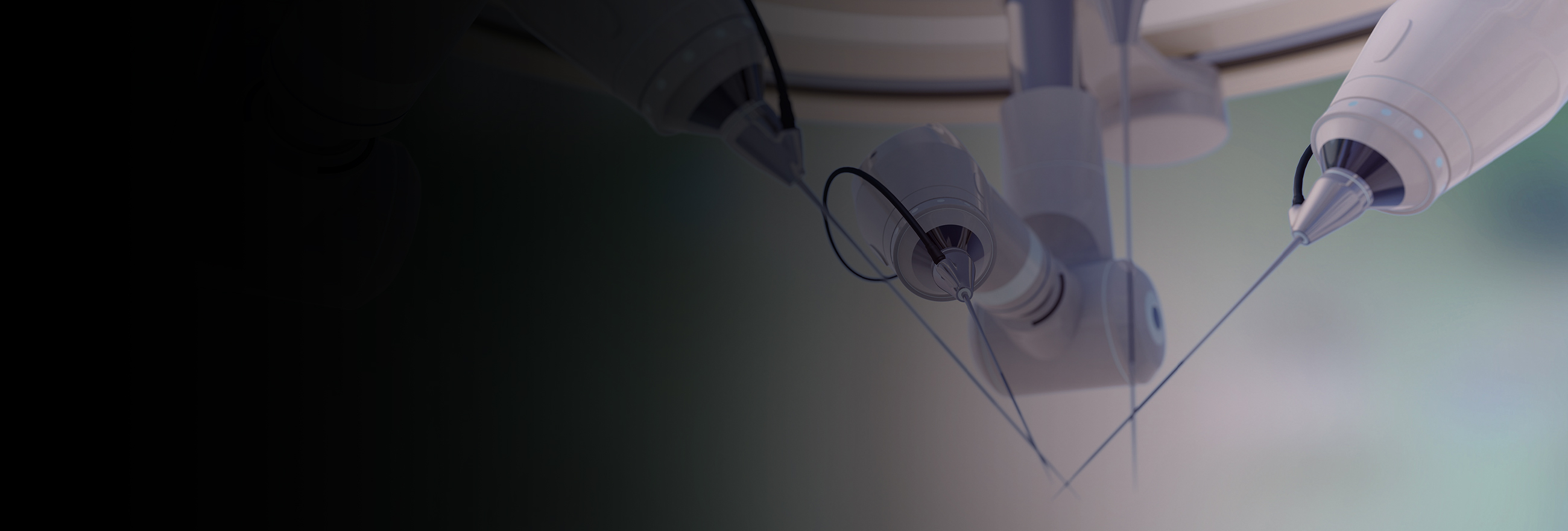
What is on the horizon for the use of data and technology in the healthcare and life sciences sectors? Where do opportunities lie, and what should participants be thinking about before making the next big move?
We provide an outlook on Health Tech trends to watch in 2023.
Partner Gunnar Sachs says...
"The commercialisation of medical robotics and technologies enabling remote medical care will give rise to novel applications of existing legal frameworks and expose areas where laws have not kept pace with the potential uses of increasingly sophisticated technology in a digitally connected world. Clear contractual arrangements as to rights, responsibilities and legal recourse will also be crucial."
Partner Steven Reese says...
"With increasing cross-border provision of products and services, identifying and navigating the relevant regulatory regimes – such as those relating to healthcare provision, medical devices, intellectual property and data use – will be critical to the success of pioneers in this area and a potential weapon in competition."
 Clifford Chance
Clifford Chance